The battle against the Coronavirus seems to get a breakthrough. Two premier drug companies have recently publicized that they achieved highly successful results from phase III trials of COVID19 vaccines. While Pfizer and partner BioNTech revealed that their vaccine would be 95 percent effective at preventing the virus infection, Moderna reported its vaccine would be 94.5 percent effective.
Both the drug majors have used the same genetically engineered approach to produce the vaccines that involve messenger RNA molecules. Pfizer has planned to produce up to 50 million doses in 2020 and up to 1.3 billion doses in 2021. And Moderna has planned to produce approximately 20 million doses in 2020 and then ramp up to 500 million, and then to one billion in 2021.
When a person receives either of the vaccines, he will need two doses to be administered three or four weeks apart.
There will be multiple steps that will be involved to deliver the vaccines from the production facilities to local hospitals and pharmacies, where the actual vaccination will take place.
According to the available information, a temperature of minus 20 degrees has to be maintained while shipping Moderna’sModerna’s vaccine, and the vaccine can be stored at that temperature for six months. Once thawed and kept in a refrigerator between two and eight degrees C, the vaccine will remain good for up to 30 days. And Pfizer’sPfizer’s vaccine must be stored at minus 70 degrees C, and the vaccine must be administered within five days after transferring to a refrigerator.
Now, let us look at the supply chain and the associated challenges:
Cold Chain
Before the vaccine gets to your nearest hospital or pharmacy, governments have to put in place the right infrastructure to transport and store safely. The vaccines need to be kept at freezing temperatures until they are administered.
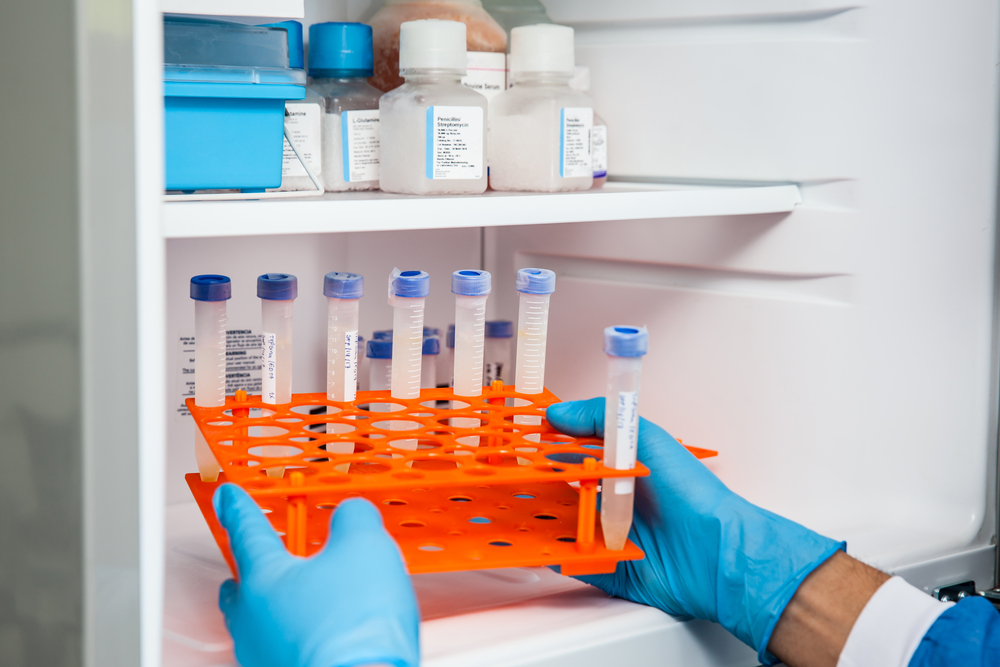
The need to transport and store the vaccines at minus degree temperature environments will pose a strong challenge for logistic companies. The vaccines will have to be transported from country to country and then from warehouses to hospitals and pharmacies in freezing temperatures.
Trucks and planes will have to be fitted with freezers to actuate the vaccines’ right transportation. Hospitals and pharmacies will require cold storage, such as ultra-low temperature freezers, to store the vaccines.
Other Essential Components
The right cold chain is only a part of the entire requirement for transporting the vaccines along the entire supply chain. There will be several essential components, such as vials, stoppers, gauze, and alcohol swabs, which must be put to use during the vaccines’ journey from production facilities to the public.
For example, vials need to be specially made to withstand minus degrees temperatures as the glass can develop cracks in such freezing temperatures.
Moreover, proper sterilization is critical for vials. Dirty vials will damage the vaccine and render it ineffective and can even harm the administered person.
Likewise, proper sterilization is also required for stoppers, syringes, and needles.
Who Will Get the Vaccine First?
Governments have to draw up a priority plan as there will be a limited supply of the vaccines at the beginning. Ideally, the plan should target the elderly for the first vaccine phase, as they are the most vulnerable section of the population.
If the elderly segment of the population is vaccinated first, it will help to reduce mortality. In the next phase, the working population can be targeted as vaccinating them can prevent the spread of infection.
However, the World Health Organization believes that the elderly and frontline health workers should be the first vaccine recipients.
It is also not difficult to figure out that Pfizer’s vaccine could go to cities as large healthcare institutions can have ultracold storage. Moderna’s vaccine could go to less densely populated areas where the appropriate cold storage facilities to store the vaccine can be available at medical facilities.
Conclusion
Given the urgency of administering the vaccine, healthcare facilities should now gear up to safely handle and store the vaccines once they arrive. It is now the time for medical facility owners to invest in cold storage units, such as ultra-low temperature freezers.